Properties of Gases
understanding matter at its simplest
chem1 virtual textbook
a reference text for General Chemistry
Stephen Lower
Simon Fraser University
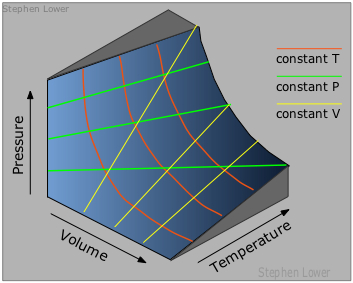
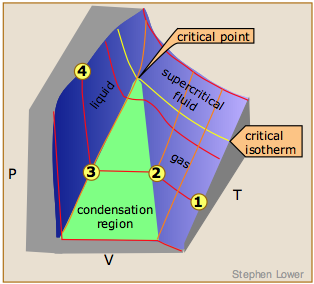
The gaseous state of matter is the only one that is based on a simple model that can be developed from first principles. As such, it serves as the starting point for the study of the other states of matter.
The Chem1 Virtual Textbook is a resource for General Chemistry aimed mainly at the first-year university level. It offers a more comprehensive, organized, and measured approach than is found in most standard textbooks. It should also be accessible to advanced high-school courses, and helpful as review material for students in more advanced courses in chemistry, biology, geology, and engineering.

Needlepoint [link] by B. Schaefer[?]