 Water has long been known to exhibit many physical properties that distinguish it from other small molecules of comparable mass. Chemists refer to these as the "anomalous" properties of water, but they are by no means mysterious; all are entirely predictable consequences of the way the size and nuclear charge of the oxygen atom conspire to distort the electronic charge clouds of the atoms of other elements when these are chemically bonded to the oxygen.
Water has long been known to exhibit many physical properties that distinguish it from other small molecules of comparable mass. Chemists refer to these as the "anomalous" properties of water, but they are by no means mysterious; all are entirely predictable consequences of the way the size and nuclear charge of the oxygen atom conspire to distort the electronic charge clouds of the atoms of other elements when these are chemically bonded to the oxygen.
Science and art come together in this painting by Taylor Mulder which portrays the water molecule - two hydrogens and one oxygen - in abstract figures enclosed in a molecular sphere. [link]
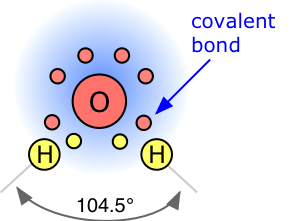 A covalent chemical bond consists of a pair of electrons shared between two atoms. In the water molecule H2O, the single electron of each H is shared with one of the six outer-shell electrons of the oxygen, leaving four electrons which are organized into two non-bonding pairs. Thus the oxygen atom is surrounded by four electron pairs that would ordinarily tend to arrange themselves as far from each other as possible in order to minimize repulsions between these clouds of negative charge. This would ordinarly result in a tetrahedral geometry in which the angle between electron pairs (and therefore the H-O-H bond angle) is 109°. However, because the two non-bonding pairs remain closer to the oxygen atom, these exert a stronger repulsion against the two covalent bonding pairs, effectively pushing the two hydrogen atoms closer together. The result is a distorted tetrahedral arrangement in which the H—O—H angle is 104.5°.
A covalent chemical bond consists of a pair of electrons shared between two atoms. In the water molecule H2O, the single electron of each H is shared with one of the six outer-shell electrons of the oxygen, leaving four electrons which are organized into two non-bonding pairs. Thus the oxygen atom is surrounded by four electron pairs that would ordinarily tend to arrange themselves as far from each other as possible in order to minimize repulsions between these clouds of negative charge. This would ordinarly result in a tetrahedral geometry in which the angle between electron pairs (and therefore the H-O-H bond angle) is 109°. However, because the two non-bonding pairs remain closer to the oxygen atom, these exert a stronger repulsion against the two covalent bonding pairs, effectively pushing the two hydrogen atoms closer together. The result is a distorted tetrahedral arrangement in which the H—O—H angle is 104.5°.
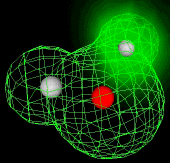
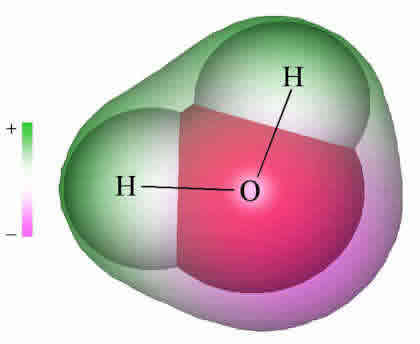
Because molecules are smaller than light waves, they cannot be observed directly, and must be "visualized" by alternative means. The two computer-generated images of the H2O molecule shown below come from calculations that model the electron distribution in molecules. The outer envelopes show the effective "surface" of the molecule as defined by the extent of the electron cloud
|
|
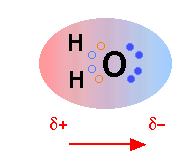
The H2O molecule is electrically neutral, but the positive and negative charges are not distributed uniformly. This is shown clearly by the gradation in color from green to purple in the image at the above right, and in the schematic diagram here. The electronic (negative) charge is concentrated at the oxygen end of the molecule, owing partly to the nonbonding electrons (solid blue circles), and to oxygen's high nuclear charge which exerts stronger attractions on the electrons. This charge displacement constitutes an electric dipole, represented by the arrow at the bottom; you can think of this dipole as the electrical "image" of a water molecule.
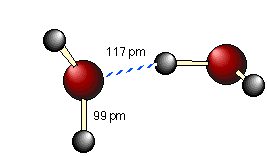 As we all learned in school, opposite charges attract, so the partially-positive hydrogen atom on one water molecule is electrostatically attracted to the partially-negative oxygen on a neighboring molecule. This process is called (somewhat misleadingly) hydrogen bonding. Notice that the hydrogen bond (shown by the dashed blue line) is somewhat longer (117 pm) than the covalent O—H bond (99 pm). This means that it is considerably weaker; it is so weak, in fact,that a given hydrogen bond cannot survive for more than a tiny fraction of a second.
As we all learned in school, opposite charges attract, so the partially-positive hydrogen atom on one water molecule is electrostatically attracted to the partially-negative oxygen on a neighboring molecule. This process is called (somewhat misleadingly) hydrogen bonding. Notice that the hydrogen bond (shown by the dashed blue line) is somewhat longer (117 pm) than the covalent O—H bond (99 pm). This means that it is considerably weaker; it is so weak, in fact,that a given hydrogen bond cannot survive for more than a tiny fraction of a second.
See here for much more about hydrogen bonding.
How chemists think about water
The nature of liquid water and how the H2O molecules within it are organized and interact are questions that have attracted the interest of chemists for many years. There is probably no liquid that has received more intensive study, and there is now a huge literature on this subject.
The following facts are well established:
- H2O molecules attract each other through the special type of dipole-dipole interaction known as hydrogen bonding
- a hydrogen-bonded cluster in which four H2Os are located at the corners of an imaginary tetrahedron is an especially favorable (low-potential energy) configuration, but...
- the molecules undergo rapid thermal motions on a time scale of picoseconds (10–12 second), so the lifetime of any specific clustered configuration will be fleetingly brief.
A variety of techniques including infrared absorption, neutron scattering, and nuclear magnetic resonance have been used to probe the microscopic structure of water. The information garnered from these experiments and from theoretical calculations has led to the development of around twenty "models" that attempt to explain the structure and behavior of water. More recently, computer simulations of various kinds have been employed to explore how well these models are able to predict the observed physical properties of water.
This work has led to a gradual refinement of our views about the structure of liquid water, but it has not produced any definitive answer. There are several reasons for this, but the principal one is that the very concept of "structure" (and of water "clusters") depends on both the time frame and volume under consideration. Thus questions of the following kinds are still open:
- How do you distinguish the members of a "cluster" from adjacent molecules that are not in that cluster?
- Since individual hydrogen bonds are continually breaking and re-forming on a picosecond time scale, do water clusters have any meaningful existence over longer periods of time? In other words, clusters are transient, whereas "structure" implies a molecular arrangement that is more enduring. Can we then legitimately use the term "clusters" in describing the structure of water?
- The possible locations of neighboring molecules around a given H2O are limited by energetic and geometric considerations, thus giving rise to a certain amount of "structure" within any small volume element. It is not clear, however, to what extent these structures interact as the size of the volume element is enlarged. And as mentioned above, to what extent are these structures maintained for periods longer than a few picoseconds?
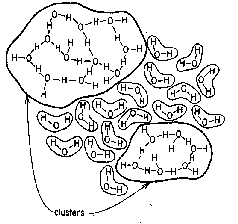 The view first developed in the 1950's that water is a collection of "flickering clusters" of varying sizes (right) has gradually been abandoned as being unable to account for many of the observed properties of the liquid. The current thinking, influenced greatly by molecular modeling simulations beginning in the 1980s, is that on a very short time scale (less than a picosecond), water is more like a "gel" consisting of a single, huge hydrogen-bonded cluster. On a 10-12-10-9 sec time scale, rotations and other thermal motions cause individual hydrogen bonds to break and re-form in new configurations, inducing ever-changing local discontinuities whose extent and influence depends on the temperature and pressure. It is quite likely that over very small volumes, localized (H2O)n polymeric clusters may have a fleeting existence, and many theoretical calculations have been made showing that some combinations are more stable than others. While this might prolong their lifetimes, it does not appear that they remain intact long enough to detect as directly observable entities in ordinary bulk water at normal pressures.
The view first developed in the 1950's that water is a collection of "flickering clusters" of varying sizes (right) has gradually been abandoned as being unable to account for many of the observed properties of the liquid. The current thinking, influenced greatly by molecular modeling simulations beginning in the 1980s, is that on a very short time scale (less than a picosecond), water is more like a "gel" consisting of a single, huge hydrogen-bonded cluster. On a 10-12-10-9 sec time scale, rotations and other thermal motions cause individual hydrogen bonds to break and re-form in new configurations, inducing ever-changing local discontinuities whose extent and influence depends on the temperature and pressure. It is quite likely that over very small volumes, localized (H2O)n polymeric clusters may have a fleeting existence, and many theoretical calculations have been made showing that some combinations are more stable than others. While this might prolong their lifetimes, it does not appear that they remain intact long enough to detect as directly observable entities in ordinary bulk water at normal pressures.
Think of liquid water of as a seething mass of H2O molecules in which hydrogen-bonded clusters are continually forming, breaking apart, and re-forming.
Theoretical models suggest that the average cluster may encompass as many as 90 H2O molecules at 0°C, so that very cold water can be thought of as a collection of ever-changing ice-like structures. At 70° C, the average cluster size is probably no greater than about 25.
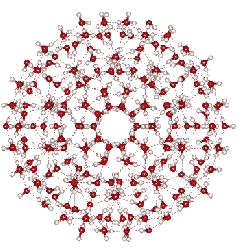 Prof. Martin Chaplin of the London South Bank University has reviewed much of the existing literature on water clustering, and has recently proposed an icosohedral clustering model in which twenty 14-molecule tetrahedral units form an icosohedron containing a total of 280 H2O units. This model is consistent with X-ray diffraction data and is able to explain all of the unusual properties of water.
Prof. Martin Chaplin of the London South Bank University has reviewed much of the existing literature on water clustering, and has recently proposed an icosohedral clustering model in which twenty 14-molecule tetrahedral units form an icosohedron containing a total of 280 H2O units. This model is consistent with X-ray diffraction data and is able to explain all of the unusual properties of water.
It must be emphasized that no stable clustered unit or arrangement has ever been isolated or identified in pure bulk liquid water. A 2006 report suggests that a simple tetrahedral arrangement is the only long-range structure that persists at time scales of a picosecond or beyond. But for an interesting alternative view, see this article by Rustum Roy.
Water clusters are of considerable interest as models for the study of water and water surfaces, and many articles on them are published every year. Some notable work reported in 2004 extended our view of water to the femtosecond time scale. The principal finding was that 80 percent of the water molecules are bound in chain-like fashion to only two other molecules at room temperature, thus supporting the prevailing view of a dynamically-changing, disordered water structure.
Liquid and solid water
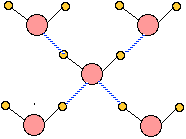 Ice, like all solids, has a well-defined structure; each water molecule is surrounded by four neighboring H2Os. two of these are hydrogen-bonded to the oxygen atom on the central H2O molecule, and each of the two hydrogen atoms is similarly bonded to another neighboring H2O.
Ice, like all solids, has a well-defined structure; each water molecule is surrounded by four neighboring H2Os. two of these are hydrogen-bonded to the oxygen atom on the central H2O molecule, and each of the two hydrogen atoms is similarly bonded to another neighboring H2O.
The hydrogen bonds are represented by the dashed lines in this 2-dimensional schematic diagram. In reality, the four bonds from each O atom point toward the four corners of a tetrahedron centered on the O atom. This basic assembly repeats itself in three dimensions to build the ice crystal.
(See this very readable article on the structure of ice)
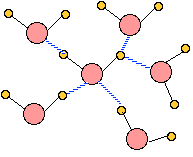 When ice melts to form liquid water, the uniform three-dimensional tetrahedral organization of the solid breaks down as thermal motions disrupt, distort, and occasionally break hydrogen bonds. The methods used to determine the positions of molecules in a solid do not work with liquids, so there is no unambiguous way of determining the detailed structure of water. The illustration here is probably typical of the arrangement of neighbors around any particular H2O molecule, but very little is known about the extent to which an arrangement like this gets propagated to more distant molecules.
When ice melts to form liquid water, the uniform three-dimensional tetrahedral organization of the solid breaks down as thermal motions disrupt, distort, and occasionally break hydrogen bonds. The methods used to determine the positions of molecules in a solid do not work with liquids, so there is no unambiguous way of determining the detailed structure of water. The illustration here is probably typical of the arrangement of neighbors around any particular H2O molecule, but very little is known about the extent to which an arrangement like this gets propagated to more distant molecules.
Below are three-dimensional views of a typical local structure of liquid water (right) and of ice (left). Notice the greater openness of the ice structure which is necessary to ensure the strongest degree of hydrogen bonding in a uniform, extended crystal lattice. The more crowded and jumbled arrangement in liquid water can be sustained only by the greater amount thermal energy available above the freezing point.
|
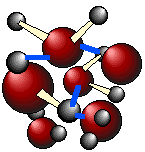 water |
 The stable arrangement of hydrogen-bonded water molecules in ice gives rise to the beautiful hexagonal symmetry that reveals itself in every snowflake.
The stable arrangement of hydrogen-bonded water molecules in ice gives rise to the beautiful hexagonal symmetry that reveals itself in every snowflake.
The anomalous properties of water
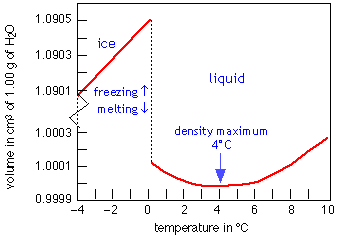
Water is almost unique among the more than 15 million known chemical substances in that its solid form is less dense than the liquid. The plot at the right shows how the volume of water varies with the temperature; the large increase (about 9%) on freezing shows why ice floats on water and why pipes burst when they freeze. The expansion between –4° and 0° is due to the formation of larger clusters. Above 4°, thermal expansion sets in as thermal vibrations of the O—H bonds becomes more vigorous, tending to shove the molecules farther apart.
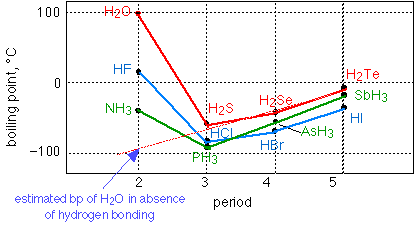 The other widely-cited anomalous property of water is its high boiling point. As this graph shows, a molecule as light as H2O "should" boil at around –90°C; that is, it should exist in the world as a gas rather than a liquid, if H-bonding were not present. Notice that H-bonding is also observed with fluorine and nitrogen.
The other widely-cited anomalous property of water is its high boiling point. As this graph shows, a molecule as light as H2O "should" boil at around –90°C; that is, it should exist in the world as a gas rather than a liquid, if H-bonding were not present. Notice that H-bonding is also observed with fluorine and nitrogen.
"Forty-one anomalies of water" — some of them rather esoteric
Surface tension and wetting
 Have you ever watched an insect walk across the surface of a pond? The water strider takes advantage of the fact that the water surface acts like an elastic film that resists deformation when a small weight is placed on it. (If you are careful, you can also "float" a small paper clip or steel staple on the surface of water in a cup.) This is all due to the surface tension of the water. A molecule within the bulk of a liquid experiences attractions to neighboring molecules in all directions,
but since these average out to zero, there is no net force on the molecule. For a molecule at the surface, the situation is quite different; it experiences forces only sideways and downward, and this is what creates the stretched-membrane effect.
Have you ever watched an insect walk across the surface of a pond? The water strider takes advantage of the fact that the water surface acts like an elastic film that resists deformation when a small weight is placed on it. (If you are careful, you can also "float" a small paper clip or steel staple on the surface of water in a cup.) This is all due to the surface tension of the water. A molecule within the bulk of a liquid experiences attractions to neighboring molecules in all directions,
but since these average out to zero, there is no net force on the molecule. For a molecule at the surface, the situation is quite different; it experiences forces only sideways and downward, and this is what creates the stretched-membrane effect.
The distinction between molecules located at the surface and those deep inside is especially prominent in H2O, owing to the strong hydrogen-bonding forces. The difference between the forces experienced by a molecule at the surface and one in the bulk liquid gives rise to the liquid's surface tension.
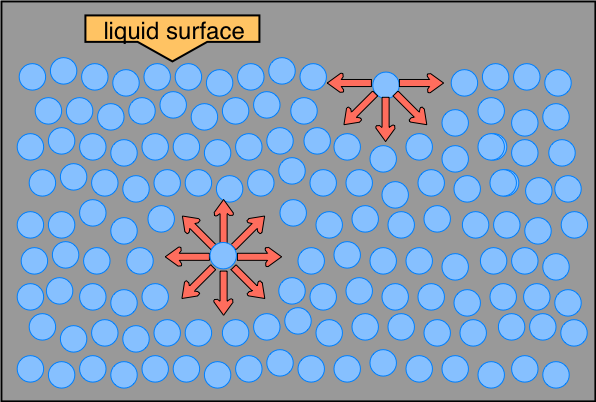 This drawing highlights two H2O molecules, one at the surface, and the other in the bulk of the liquid. The surface molecule is attracted to its neighbors below and to either side, but there are no attractions pointing in the 180° solid angle angle above the surface.
As a consequence, a molecule at the surface will tend to be drawn into the bulk of the liquid. But since there must always be some surface, the overall effect is to minimize the surface area of a liquid. The geometric shape that has the smallest ratio of surface area to volume is the sphere, so very small quantities of liquids tend to form spherical drops. As the drops get bigger, their weight deforms them into the typical tear shape.
This drawing highlights two H2O molecules, one at the surface, and the other in the bulk of the liquid. The surface molecule is attracted to its neighbors below and to either side, but there are no attractions pointing in the 180° solid angle angle above the surface.
As a consequence, a molecule at the surface will tend to be drawn into the bulk of the liquid. But since there must always be some surface, the overall effect is to minimize the surface area of a liquid. The geometric shape that has the smallest ratio of surface area to volume is the sphere, so very small quantities of liquids tend to form spherical drops. As the drops get bigger, their weight deforms them into the typical tear shape.
Wetting
Take a plastic mixing bowl from your kitchen, and splash some water around in it. You will probably observe that the water does not cover the inside surface uniformly, but remains dispersed into drops. The same effect is seen on a dirty windshield; turning on the wipers simply breaks hundreds of drops into thousands. By contrast, water poured over a clean glass surface will wet it, leaving a uniform film.
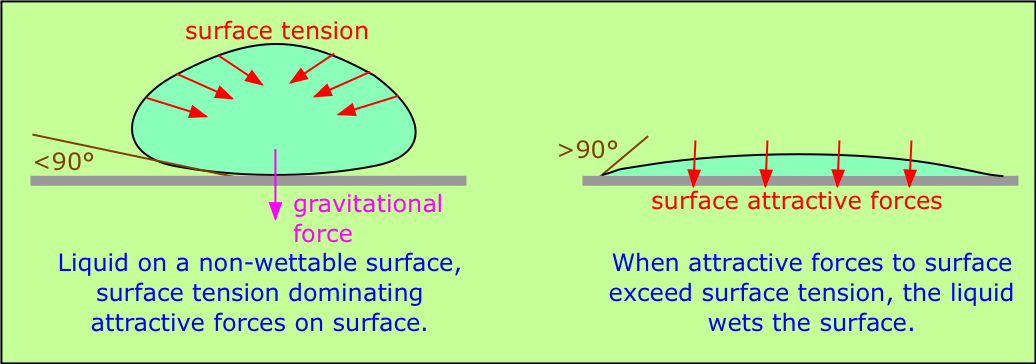
When a liquid is in contact with a solid surface, its behavior depends on the relative magnitudes of the surface tension forces and the attractive forces between the molecules of the liquid and of those comprising the surface. If an H2O molecule is more strongly attracted to its own kind, then surface tension will dominate, increasing the curvature of the interface. This is what happens at the interface between water and a hydrophobic surface such as a plastic mixing bowl or a windshield coated with oily material. A clean glass surface, by contrast, has -OH groups sticking out of it which readily attach to water molecules through hydrogen bonding; this causes the water to spread out evenly over the surface, or to wet it. A liquid will wet a surface if the angle at which it makes contact with the surface is more than 90°. The value of this contact angle can be predicted from the properties of the liquid and solid separately.
If we want water to wet a surface that is not ordinarily wettable, we add a detergent to the water to reduce its surface tension. A detergent is a special kind of molecule in which one end is attracted to H2O molecules but the other end is not; the latter ends stick out above the surface and repel each other, cancelling out the surface tension forces due to the water molecules alone.
"Pure" water
To a chemist, the term "pure" has meaning only in the context of a particular application or process. The distilled or de-ionized water we use in the laboratory contains dissolved atmospheric gases and occasionally some silica, but their small amounts and relative inertness make these impurities insignificant for most purposes. When water of the highest obtainable purity is required for certain types of exacting measurements, it is commonly filtered, de-ionized, and triple-vacuum distilled. But even this "chemically pure" water is a mixture of isotopic species: there are two stable isotopes of both hydrogen (H1 and H2, often denoted by D) and oxygen (O16 and O18) which give rise to combinations such as H2O18, HDO16, etc., all of which are readily identifiable in the infrared spectra of water vapor. (Interestingly, the amount of the rare isotopes of oxygen and hydrogen in water varies enough from place to place that it is now possible to determine the age and source of a particular water sample with some precision.) And to top this off, the two hydrogen atoms in water contain protons whose magnetic moments can be parallel or antiparallel, giving rise to ortho- and para-water, respectively. The two forms are normally present in a o/p ratio of 3:1.
It has recently been found (Langmuir 2003, 19, 6851-6856) that freshly distilled water takes a surprisingly long time to equilibrate with the atmosphere, that it undergoes large fluctuations in pH and redox potential, and that these effects are greater when the water is exposed to a magnetic field. The reasons for this behavior are not clear, but one possibility is that dissolved O2 molecles, which are paramagnetic, might be involved.
Drinking water
Our ordinary drinking water, by contrast, is never chemically pure, especially if it has been in contact with sediments. Groundwaters (from springs or wells) always contain ions of calcium and magnesium, and often iron and manganese as well; the positive charges of these ions are balanced by the negative ions carbonate/bicarbonate, and occasionally some chloride and sulfate. Groundwaters in some regions contain unacceptably high concentrations of naturally-occuring toxic elements such as selenium and arsenic.
One might think that rain or snow would be exempt from contamination, but when water vapor condenses out of the atmosphere it always does so on a particle of dust which releases substances into the water, and even the purest air contains carbon dioxide which dissolves to form carbonic acid. Except in highly polluted atmospheres, the impurities picked up by snow and rain are too minute to be of concern.
Various governments have established upper limits on the amounts of contaminants allowable in drinking water; the best known of these are the U.S. EPA Drinking Water Standards.
One occasionally hears that waters containing unusually small amounts of minerals, and distilled water particularly, are unhealthy because they "leach out" required minerals from the body. Such claims are unfounded; the fact is that cell walls contain one-way channels that utilize metabolic energy to actively transport needed ions against what is often a substantial concentration gradient. (See here more on this.)
Structured water and biowater
As we explained above, bulk liquid water consists of a seething mass of various-sized chain-like groups and that flicker in and out of existence on a time scale of picoseconds. But in the vicinity of a solid surface or of another molecule or ion that possesses an unbalanced electric charge, water molecules can become oriented and sometimes even bound into relatively stable structures.
Water in ionic hydration shells
Water molecules interact strongly with ions, which are electrically-charged atoms or molecules. Dissolution of ordinary salt (NaCl) in water yields a solution containing the ions Na+ and Cl –. Owing to its high polarity, the H2O molecules closest to the dissolved ion are strongly attached to it, forming what is known as the inner or primary hydration shell. Positively-charged ions such as Na+ attract the negative (oxygen) ends of the H2O molecules, as shown in the diagram below. The ordered structure within the primary shell creates, through hydrogen-bonding, a region in which the surrounding waters are also somewhat ordered; this is the outer hydration shell, or cybotactic region.
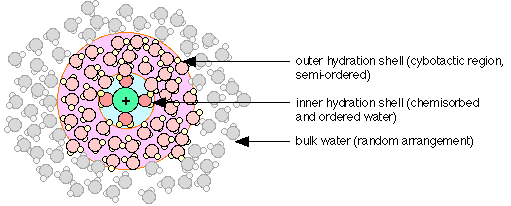
Some recent experiments have revealed a degree of covalent bonding between the d-orbitals of transition metal ions and the oxygen atoms of water molecules in the inner hydration shell.
Bound water in biological systems
It has long been known that the intracellular water very close to any membrane or organelle (sometimes called vicinal water) is organized very differently from bulk water, and that this structured water plays a significant role in governing the shape (and thus biological activity) of large folded biopolymers. It is important to bear in mind, however, that the structure of the water in these regions is imposed solely by the geometry of the surrounding hydrogen bonding sites.
 Water can hydrogen-bond not only to itself, but also to any other molecules that have -OH or -NH2 units hanging off of them. This includes simple molecules such as alcohols, surfaces such as glass, and macromolecules such as proteins. The biological activity of proteins (of which enzymes are an important subset) is critically dependent not only on their composition but also on the way these huge molecules are folded; this folding involves hydrogen-bonded interactions with water, and also between different parts of the molecule itself. Anything that disrupts these intramolecular hydrogen bonds will denature the protein and destroy its biological activity. This is essentially what happens when you boil an egg; the bonds that hold the egg white protein in its compact folded arrangement break apart so that the molecules unfold into a tangled, insoluble mass which, like Humpty Dumpty, cannot be cannot be restored to their original forms. Note that hydrogen-bonding need not always involve water; thus the two parts of the DNA double helix are held together by H—N—H hydrogen bonds.
Water can hydrogen-bond not only to itself, but also to any other molecules that have -OH or -NH2 units hanging off of them. This includes simple molecules such as alcohols, surfaces such as glass, and macromolecules such as proteins. The biological activity of proteins (of which enzymes are an important subset) is critically dependent not only on their composition but also on the way these huge molecules are folded; this folding involves hydrogen-bonded interactions with water, and also between different parts of the molecule itself. Anything that disrupts these intramolecular hydrogen bonds will denature the protein and destroy its biological activity. This is essentially what happens when you boil an egg; the bonds that hold the egg white protein in its compact folded arrangement break apart so that the molecules unfold into a tangled, insoluble mass which, like Humpty Dumpty, cannot be cannot be restored to their original forms. Note that hydrogen-bonding need not always involve water; thus the two parts of the DNA double helix are held together by H—N—H hydrogen bonds.
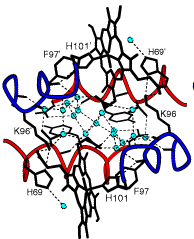
The picture above, taken from the work of William Royer Jr. of the U. Mass. Medical School, shows the water structure (small green circles) that exists in the space between the two halves of a kind of dimeric hemoglobin. The thin dotted lines represent hydrogen bonds. Owing to the geometry of the hydrogen-bonding sites on the heme protein backbones, the H2O molecules within this region are highly ordered; the local water structure is stabilized by these hydrogen bonds, and the resulting water cluster in turn stabilizes this particular geometric form of the hemoglobin dimer. More diagrams, with commentary, can be found on Prof. Royer's Web site.
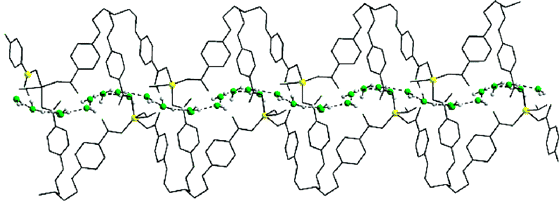
In 2003, some chemists in India found (Inorg. Chem. 44(4) pp 816 - 818) that a suitable molecular backbone (above) can even cause water molecules to form a "thread" that can snake its way though the more open space of the larger molecules. What all of these examples show is that water can have highly organized local structures when it interacts with molecules capable of imposing these structures on the water.
"Clustered", "Unclustered" and other structure-altered waters
 The "alternative" health market is full of goofy products which purport to alter the structure of water by stabilizing groups of H2O molecules into permanent clusters of 4-8 molecules, or alternatively, to break up what they claim are the larger clusters (usually 10-15 molecules) that they say normally exist in water. The object in either case is to promote the flow of water into the body's cells ("cellular hydration"). This is of course utter nonsense; there is no credible scientific evidence for any of these claims, many of which verge on the bizarre. There are even some scientifically absurd U.S. Patents for the manufacture of so-called "Clustered Water™". At least 20 nostrums of this kind are offered to the scientifically-naive public through hundreds of Web sites and late-night radio "infomercials". None of these claims is supported by credible evidence.
The "alternative" health market is full of goofy products which purport to alter the structure of water by stabilizing groups of H2O molecules into permanent clusters of 4-8 molecules, or alternatively, to break up what they claim are the larger clusters (usually 10-15 molecules) that they say normally exist in water. The object in either case is to promote the flow of water into the body's cells ("cellular hydration"). This is of course utter nonsense; there is no credible scientific evidence for any of these claims, many of which verge on the bizarre. There are even some scientifically absurd U.S. Patents for the manufacture of so-called "Clustered Water™". At least 20 nostrums of this kind are offered to the scientifically-naive public through hundreds of Web sites and late-night radio "infomercials". None of these claims is supported by credible evidence.
Does water have "memory"?
According to modern-day proponents of homeopathy, it must. Homeopathic remedies are made by diluting solutions of various substances so greatly that not even a single molecule of the active substance can be expected to be present in the final medication. Now that even the homeopaths have come to accept this fact, they explain that the water somehow retains the "imprint" or "memory" of the original solute.
In 1985, the late Jacques Benveniste, a French biologist, conducted experiments that purported to show that a certain type of cellular immune response could be brought about by an anti-immunoglobulin agent that had been diluted to such an extent that it is highly unlikely that even one molecule of this agent remained in the aqueous solution. He interpreted this to indicate that water could somehow retain an impression, or "memory", of a solute that had been diluted out of existence. This result was immediately taken by believers in homeopathy as justification for their dogma that similarly diluted remedies could be effective as alternative medical agents. The consensus among chemists is that any temporary disruption of the water structure by a dissolved agent would disappear within a fraction of a second after its removal by dilution, owing to the vigorous thermal motions of the water molecules. Benveniste's results have never been convincingly replicated by other scientists (see here for a recent summary).
References
The mystery, art and science of water. This site provides a wonderful view of water in all the many ways it impacts upon the multiple facets of our culture. Highly recommended.
Water Structure and Properties is a Web site developed by Martin Chaplin at South Bank University in England. It is a scientifically sound, well laid-out collection of articles on water and its structure which should answer any of your questions.
Does hot water freeze faster than cold water? Yes, this can happen under the right conditions. Brief explanation, more complete explanation. See also Warm water vibrates for a longer time.
Water Clusters. K. Liu, J.D. Cruzan, and R.J. Saykally. Science 1996 929-993 - A summary of experimental data on the structures, energetics and dynamics of small clusters, and comparisons with theoretical predictions.
"Water Buckyballs": Chemical, catalytic and cosmic implications. This rather technical paper by Keith Johnson of MIT explores the quantum theory and far-i.r. spectra of water clusters and speculates on their role in cosmochemistry.
{The structure of ideal liquid water} - a well-organized but rather technical Web site by Gregory Moreno. It includes an extensive bibliography of scientific articles on water structure from 1915 through 1992.
Cell-associated water. W. Drost-Hansen, J. Clegg, ed. Academic Press, 1979. This is a collection of 11 papers given at a conference in 1976. It predates the availability of modern laser-based methods of examining water structure, but contains a lot of indirect observations and NMR results.
Water on earth: the hydrosphere and the oceans - this site, from the Author's former course in Environmental Chemistry, presents a general survey.
Why is water blue? It's all about O-H bond stretching! A more technical site. See also this nicely illustrated NASA article Where is the ocean bluest?
And finally, for a darker view of water, see the Ban DHMO page